
A lateral flow immunoassay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. The sample material can be obtained by using a nasal swab during the acute phase of the infection.
The FORA COVID-19 Antigen Rapid Test is clinically validated for testing with nasal and nasopharyngeal sample.
Benefits
| PCR Test Result | |||||
|---|---|---|---|---|---|
| Positive | Negative | Subtotal | |||
| FORA COVID-19 Antigen Rapid Test | |||||
| Positive | 93 | 1 | 94 | ||
| Negative | 7 | 141 | 148 | ||
| Subtotal | 100 | 142 | 242 | ||
| Sensitivity | 95.7% (95% CI: 89.3% – 98.3%) | ||||
| Specificity | 99.3% (95% CI: 96.1%-99.9% | ||||

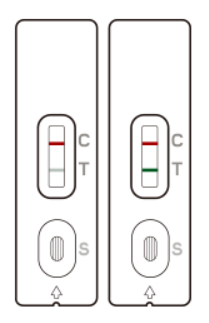
Two colored lines appear: One line in the control line region (C) and one in the test line region (T). The result is positive regardless of the intensity of the T-line. The patient is most likely INFECTED with the COVID-19 infection*.
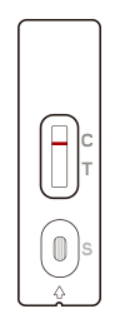
A line in the Control line region (C) appears. No line in the Test line region (T). The patient is most likely NOT INFECTED with the COVID-19 infection*.
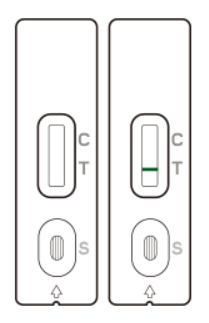
If no line appears in the Control zone (C), the test is invalid.



| Test Principle | Lateral Flow Chromatographic Immunoassay |
| Target Antigen | SARS-CoV-2 Nucleocapsid Protein |
| Sample Type | Fresh Nasal Specimen |
| Limit of Detection (LoD) | 1.26 x 10^2 TCID50 per mL |
| Storage Condition | 2~30°C |
| Cross-reactivity & Interferences | Viruses, Bacteria and Interferences tested do not cross-react or interfere |
| Reaction Time | 15 minutes |