
A lateral flow immunoassay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. The sample material is obtained by using a nasopharyngeal swab during the acute phase of the infection.
Benefits
Clinical performance of FORA COVID-19 Antigen Rapid Test was determined by testing 103 positive and 268 negative specimens for SARS CoV-2 antigen (Ag) to have a sensitivity of 94.2% (95% CI: 87.9% – 97.3%) and specificity of 99.6% (95% CI: 97.9%-99.9%).
| PCR Test Result | |||||
|---|---|---|---|---|---|
| Positive | Negative | Subtotal | |||
| FORA COVID-19 Antigen Rapid Test | |||||
| Positive | 97 | 1 | 98 | ||
| Negative | 6 | 267 | 273 | ||
| Subtotal | 103 | 268 | 371 | ||
| Sensitivity | 94.2% (95% CI: 87.9% – 97.3%) |
||||
| Specificity | 99.6% (95% CI: 97.9%-99.9% |
||||

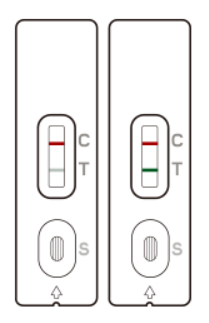
Two colored lines appear: One line in the control line region (C) and one in the test line region (T). The result is positive regardless of the intensity of the T-line.
The patient is most likely INFECTED with the COVID-19 infection*.
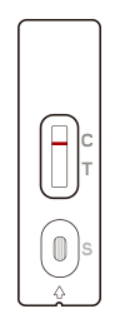
A line in the Control line region (C) appears. No line in the Test line region (T).
The patient is most likely NOT INFECTED with the COVID-19 infection*.
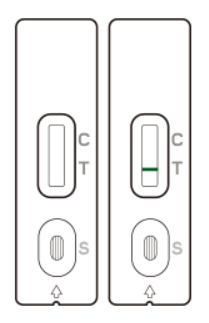
If no line appears in the Control zone (C), the test is invalid.

*No test is ever perfect. All tests occasionally result in false-positive or false-negative results. Sometimes the results are not definitive. For this and other reasons, results should always be reviewed by healthcare professionals.
Reference: CDC(2020). Guidance on Interpreting COVID-19 Test Results.

20-Test Kits / Box:


| Test Principle | Lateral Flow Chromatographic Immunoassay |
| Target Antigen | SARS-CoV-2 Nucleocapsid Protein |
| Sample Type | Fresh Nasopharyngeal Swab Specimen |
| Limit of Detection (LoD) | 1.26 x 10^2 TCID50 per mL |
| Storage Condition | 2~30°C |
| Cross-reactivity & Interferences | Viruses, Bacteria and Interferences tested do not cross-react or interfere |
| Reaction Time | 15 minutes |